
The Micro-Environmental Control Systems Your Samples Can’t Live Without
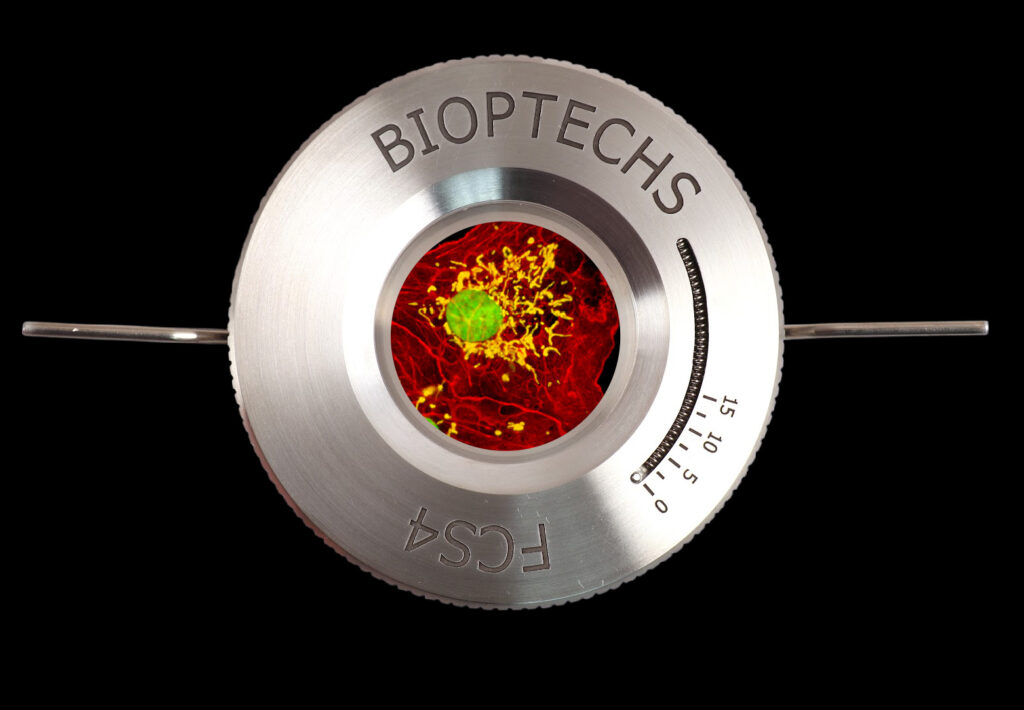
Bioptechs offers technical support and objective information about all aspects of micro-environmental control for imaging microscopy. Whether you need help identifying what instrumentation would best suit your protocol or advice on techniques and insights our experienced team is available to help.
For a general overview of micro-environmental control options on the market checkout this article in BioPhotonics.
If you would like to speak to a member of our team about your specific application we are here to help.




























3560 Beck Road
Butler, PA 16002
U.S.A.
sales@bioptechs.com
1-877-877-LIVE-CELL (548-3235)
(United States & Canada)
Direct: +1-724-282-7145
(United States & International)